LEARNING OBJECTIVES:
- What are isotopes?
- What are the similarities and differences between different isotopes of elements?
- To learn about the isotopes of Hydrogen, Chlorine, Carbon, and Uranium.
ISOTOPES:
Isotopes are the atom of an element that have the same atomic number but different atomic mass. Different elements such as Carbon, Hydrogen, Potassium, Chlorine, and Uranium exist in multiple naturally occurring isotopes. Carbon exists as three isotopes C-12, C-13, and C-14.
As the definition suggests that they have different mass so they will have different physical properties and they have the same atomic number this means the number of proton and neutron remains the same and so the chemical properties are identical. Therefore we can now have a deeper look into the isotopes that have some similarities as well as differences.
Similarities:
- Isotopes of an element have the same atomic number.
- Isotopes of an element have the same number of protons and electrons.
- Isotopes of an element have the same chemical properties.
Differences:
- Isotopes of an element have different numbers of neutrons.
- Isotopes of an element have different atomic masses.
- Isotopes of an element have different physical properties.
Now we will discuss isotopes of Hydrogen, Chlorine, Carbon, and Uranium.
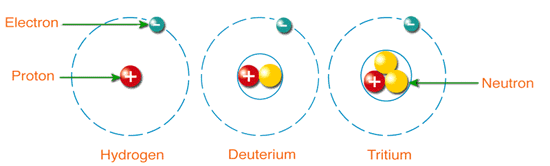
Isotopes of Hydrogen:
Hydrogen is the first element of the periodic table. There are three naturally occurring isotopes of hydrogen while there are 4 more isotopes of hydrogen H-4, H-5, H-6, and H-7. These four isotopes are synthetic.
- Hydrogen-1 (Protium)
- Hydrogen-2 (Deuterium)
- Hydrogen-3 (Tritium)
Protium (H-1):
- Atomic Number: 1
- Atomic Mass: 1
- Number of electrons: 1
- Number of protons: 1
- Number of neutrons: 0
- Natural Abundance: 99.98%
Deuterium (H-2):
- Atomic Number: 1
- Atomic Mass: 2
- Number of electrons: 1
- Number of protons: 1
- Number of neutrons: 1
- Natural Abundance: 0.015%
Tritium (H-3):
- Atomic Number: 1
- Atomic Mass: 3
- Number of electrons: 1
- Number of protons: 1
- Number of neutrons: 2
- Natural Abundance: Radioactive, Rare
Isotopes of Chlorine:
Chlorine is a member of the halogen family of the periodic table. There are 2 naturally
occurring stable isotopes of chlorine Cl-35 and Cl-37 but there is a total of 25 isotopes of chlorine.
- Chlorine-35
- Chlorine-37
Chlorine Cl-35:
- Atomic Number: 17
- Atomic Mass: 35
- Number of electrons: 17
- Number of protons: 17
- Number of neutrons: 18
- Natural Abundance: 76%
Chlorine Cl-37:
- Atomic Number: 17
- Atomic Mass: 37
- Number of electrons: 17
- Number of protons: 17
- Number of neutrons: 20
- Natural Abundance: 24%
Isotopes of Carbon:
Carbon belongs to group IV A of the periodic table. There are 15 isotopes of Carbon among these we will discuss these three isotopes of carbon.
- Carbon-12
- Carbon-13
- Carbon-14
Carbon C-12:
- Atomic Number: 6
- Atomic Mass: 12
- Number of electrons: 6
- Number of protons: 6
- Number of neutrons: 6
- Natural Abundance: 98.9%
Carbon C-13:
- Atomic Number: 6
- Atomic Mass: 13
- Number of electrons: 6
- Number of protons: 6
- Number of neutrons: 7
- Natural Abundance: 1.1%
Carbon C-14:
- Atomic Number: 6
- Atomic Mass: 14
- Number of electrons: 6
- Number of protons: 6
- Number of neutrons: 8
- Natural Abundance: Trace
Isotopes of Uranium:
Naturally, there are three isotopes of uranium. Those three are given below
- Uranium-234
- Uranium-235
- Uranium-238
Uranium U-234:
- Atomic Number: 92
- Atomic Mass: 234
- Number of electrons: 92
- Number of protons: 92
- Number of neutrons: 142
- Natural Abundance: 99.98%
Uranium U-235:
- Atomic Number: 92
- Atomic Mass: 235
- Number of electrons: 92
- Number of protons: 92
- Number of neutrons: 143
- Natural Abundance: 0.72%
Uranium U-238:
- Atomic Number: 92
- Atomic Mass: 238
- Number of electrons: 92
- Number of protons: 92
- Number of neutrons: 146
- Natural Abundance: 99.27%
SUMMARY:
- Isotopes are atoms of an element that have the same atomic number, the same number of protons and electrons, and have the same chemical properties, but have different atomic mass, different numbers of neutrons, and different physical properties.
- Hydrogen has three main isotopes H-1, H-2, H-3.
- Carbon has three main isotopes i.e. C-12, C-13, C-14.
- Chlorine has two main isotopes i.e. Cl-35, Cl-37.
- Uranium has three main isotopes i.e. U-234, U-235, U-238