Introduction:
The elements of group 17 are known as halogens. (Greek: salt former) and includes fluorine (F), Chlorine (Cl), Bromine (Br), Iodine (I), and Astatine (As). Astatine is the least common halogen and is, in fact, a decaying radioactive element that has similar properties to these elements. It has one electron less in the valence shell so it needs one electron to complete its octet.
Group trends:
Atomicity and physical state:
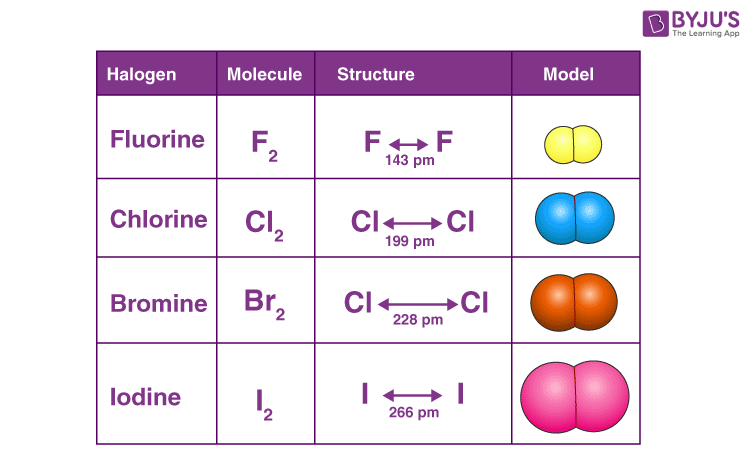
The elemental halogens exist as a diatomic molecules containing single covalent bonds. These discrete molecules are held together by weak Van der Waal's forces. The Van der wall forces increase with the increase of atomic size and molecular mass down the group because of greater polarization from fluorine to iodine. F2 is yellow gas, Cl2 is a greenish gas, Br2 is a reddish-brown liquid and I2 is a shinny black solid at room temperature.
Electronegativity:
All the halogens are highly electronegative. These values decrease gradually from F and I. Fluorine is the most electronegative element and forms hydrogen bonding in HF which shows associated molecules. The high electronegativity indicates that they attract electrons nearly by all binary compounds containing a metal and halogen.
AgF > AgCl > AgBr > AgI
Dissociation Energy:
The dissociation ion energy of X2 molecules decrease from Cl2 > Br2 > I2. But F2 has an exceptional case having a low value of dissociation energy. This is due to the repulsive forces among the lone pairs of fluorine. The following is the order of bond dissociation energy
Cl2 > Br2 > > F2 > I2
Reduction potential:
The high standard reduction potential indicates that fluorine, chlorine, and bromine are strong oxidizing agents, while iodine is a mild oxidizing agent. The oxidizing power decrease with the increase of atomic number.
F > Cl > Br > I
Thus chlorine can oxidize both Br and Iodine but not fluorine.
The following displacement reactions can occur
\[Cl_{2}\; + \;2Br^{-1}\; \rightarrow \; Br_{2}\; +\; 2Cl^{-1}\]
Melting and boiling points:
The melting and boiling points increase from fluorine to iodine because of the stronger interactions between molecules of the larger atoms.
Oxidizing state:
When a halogen atom is in its ground state it combines with an element of a lesser electronegative atom than it, shows (-1) oxidation state. On the other hand, when it combines with more electronegative elements it exhibits (+1) since F is the most electronegative it always shows (-1) oxidation state.
Reactivity:
All the halogens are very reactive and react with metals and non-metals. F is the most reactive due to the high electronegative, small size, and low dissociation energy of the F-F bond. Fluorine always displaces other halogens from their salts.
Formation of Oxyacids:
Fluorine can't form an oxyacid but other halogens form oxy-acids.
There are four types:
- Hypohalous acids (HXO)
- Hylous acids (HXO2)
- Halic acids (HXO3)
- Pertallic acid (HXO4)
Tags
Inorganic Chemistry