Abstract:
In this experiment, various chemical tests were conducted to analyze the presence of different macromolecules in food substances. Benedict’s test was used to detect reducing sugars, iodine test for starch, spot test and emulsion test for fats, and Biuret test for proteins in solutions.
Introduction:
Food tests are essential in determining the presence of specific macromolecules in food substances. These tests are based on the chemical reactions between certain reagents and the target molecules, resulting in characteristic color changes or precipitate formations. The experiment aimed to detect reducing sugars, starch, fats, and proteins using Benedict’s test, iodine test, spot test, emulsion test, and Biuret test, respectively.
Details of the Experiment:
Procedure:
- Benedict’s Test for Reducing Sugars:
- Prepare a solution of the food sample.
- Add an equal volume of Benedict's reagent to the solution.
- Heat the mixture in a boiling water bath for 5 minutes.
- Observe the color change, indicating the presence of reducing sugars.
- Iodine Test for Starch:
- Prepare a solution of the food sample.
- Add a few drops of iodine solution to the sample.
- Observe the color change from brown to blue-black, indicating the presence of starch.
- Spot Test and Emulsion Test for Fat:
- Prepare a solution of the food sample.
- Spot a small amount of the sample on a filter paper and allow it to dry.
- Observe the transparency of the spot against light, indicating the presence of fats.
- For the emulsion test, shake the food sample vigorously with water in a test tube.
- Observe the formation of an emulsion layer, indicating the presence of fats.
- Biuret Test for Protein in Solution:
- Prepare a solution of the food sample.
- Add Biuret reagent to the sample and mix well.
- Observe the color change to violet, indicating the presence of proteins.
Observations and Calculations:
Observations for each test were recorded. Calculations were not applicable for qualitative tests like Benedict’s test, iodine test, spot test, emulsion test, and Biuret test.
 |
| Biurette test for reducing sugars |
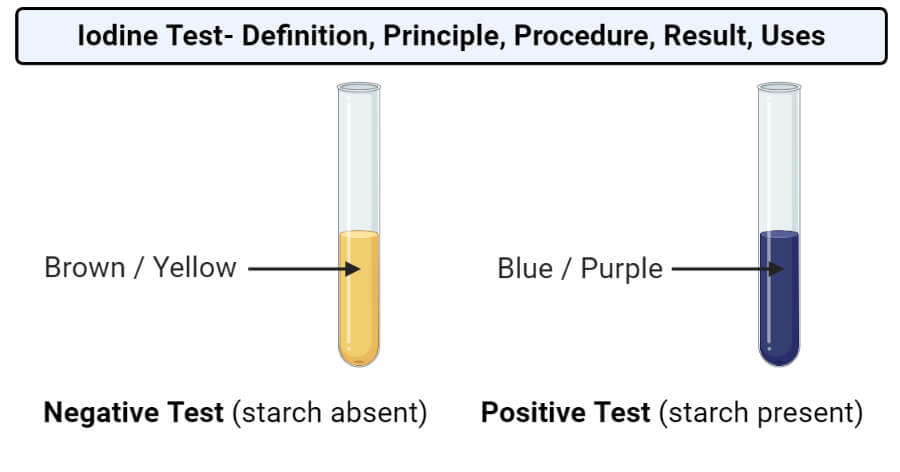 |
| Iodine test for starch |
 |
| Spot test and emulsion test for fat |
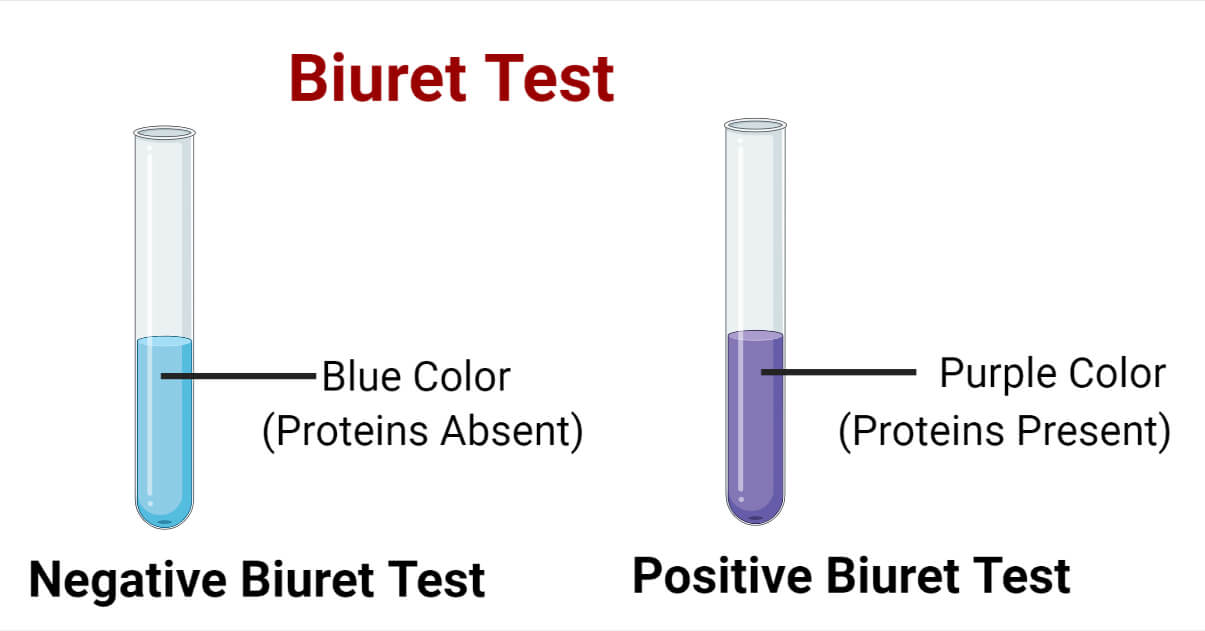 |
| Biuret test for protein in solution |
Conclusion:
The experiment successfully demonstrated the presence of reducing sugars, starch, fats, and proteins in the given food samples. Benedict’s test, iodine test, spot test, emulsion test, and Biuret test proved to be effective in detecting these macromolecules.
Precautions:
- Handle chemical reagents with care and follow safety protocols.
- Ensure proper labeling of all samples and reagents.
- Perform tests in a well-ventilated area.
- Use clean and dry glassware for accurate results.
- Dispose of chemical waste properly after the experiment.
Short Questions with Answers:
-
What is the purpose of Benedict’s test?
The purpose of Benedict’s test is to detect the presence of reducing sugars in a solution.
-
How does Benedict’s test work?
Benedict’s reagent reacts with reducing sugars in an alkaline environment, forming a colored precipitate.
-
What color indicates a positive result in Benedict’s test?
A positive result in Benedict’s test is indicated by a color change from blue to green, yellow, orange, or red, depending on the concentration of reducing sugar.
-
Why is iodine used in the iodine test?
Iodine is used in the iodine test to detect the presence of starch in a solution.
-
What color change indicates a positive result in the iodine test?
A positive result in the iodine test is indicated by a color change from yellow-brown to blue-black.
-
What is the purpose of the spot test?
The spot test is used to detect the presence of fats or lipids in a solution.
-
How does the spot test work?
In the spot test, a sample is spotted onto a piece of paper, and the appearance of a translucent spot indicates the presence of fats or lipids.
-
What is the emulsion test used for?
The emulsion test is used to confirm the presence of fats or lipids in a solution.
-
Describe the procedure of the emulsion test.
In the emulsion test, the sample is mixed with ethanol and water and shaken vigorously. The formation of a milky-white emulsion indicates the presence of fats or lipids.
-
What is the Biuret test?
The Biuret test is used to detect the presence of proteins in a solution.
-
How does the Biuret test work?
In the Biuret test, copper(II) ions in the Biuret reagent complex with peptide bonds in proteins, forming a purple-colored complex.
-
What color indicates a positive result in the Biuret test?
A positive result in the Biuret test is indicated by a color change from blue to purple.
-
Explain the importance of maintaining specific pH levels in food tests.
Specific pH levels are important in food tests because they optimize the conditions for chemical reactions to occur, ensuring accurate results.
-
What precautions should be taken while performing food tests?
Precautions include wearing appropriate safety gear, handling chemicals carefully, ensuring cleanliness of equipment, and following proper disposal procedures for chemical waste.
-
How can false positives or negatives be minimized in food tests?
False positives or negatives can be minimized by conducting control tests, ensuring proper calibration of equipment, and carefully following standardized procedures.
-
Discuss potential sources of error in food testing experiments.
Potential sources of error include contamination of samples, variations in temperature or pH, incomplete reactions, and human error in measurement or observation.
-
What are the applications of food testing in various industries?
Food testing is used in industries such as agriculture, food processing, quality control, and research to ensure the safety, quality, and nutritional content of food products.
-
How can the results of food tests be interpreted?
The results of food tests can be interpreted based on color changes, precipitate formation, or other observable changes, indicating the presence or absence of specific compounds in the tested sample.
-
What are the limitations of food testing methods?
Limitations may include the inability to detect certain compounds, interference from other substances, and the need for specialized equipment or expertise.
-
Discuss the significance of food testing in relation to human health.
Food testing plays a crucial role in ensuring the safety and nutritional value of food consumed by humans, helping to prevent foodborne illnesses and ensuring compliance with regulatory standards.
-
How can food testing contribute to environmental sustainability?
By detecting contaminants or pollutants in food products, food testing can help identify sources of environmental pollution and support efforts to mitigate environmental impact in food production and processing.
Multiple Choice Questions:
Question 1: Benedict’s Test
Explain the procedure of Benedict’s test for reducing sugar. Provide the color changes observed and the chemical reaction involved.
- What are the reagents used in Benedict’s test?
- Describe the appearance of the solution before and after the test.
- State the color changes indicating the presence of reducing sugar.
- Write the chemical equation for the reaction that occurs during Benedict’s test.
- How can you differentiate between positive and negative results in Benedict’s test?
Answer: (a) Copper sulfate and sodium carbonate. (b) Before the test, the solution is blue. After the test, it turns from blue to green, yellow, orange, or red depending on the concentration of reducing sugar. (c) The color changes from blue to green, yellow, orange, or red. (d) Cu²⁺ ions from copper sulfate are reduced to Cu⁺ ions by the aldehyde or ketone groups of reducing sugars, forming a brick-red precipitate of copper(I) oxide. (e) Positive result shows a color change to green, yellow, orange, or red, indicating the presence of reducing sugar, while negative result remains blue.
Question 2: Iodine Test
Explain the iodine test for starch. Discuss the color changes and the significance of this test.
- What is the reagent used in the iodine test?
- Describe the appearance of the solution before and after the test.
- State the color changes indicating the presence of starch.
- What is the chemical reaction involved in the iodine test?
- Explain the significance of the iodine test in identifying starch.
Answer: (a) Iodine solution (iodine dissolved in potassium iodide). (b) Before the test, the solution is brown. After the test, it turns from brown to blue-black. (c) The color changes from brown to blue-black. (d) Iodine forms a complex with starch molecules, resulting in the blue-black coloration. (e) The iodine test is significant in identifying starch as it provides a rapid and sensitive qualitative test for its presence, commonly used in food science and biology experiments.
Question 3: Spot Test
Discuss the spot test for fats and oils. Explain the principle and procedure involved.
- What is the principle behind the spot test for fats?
- Describe the procedure of the spot test.
- Explain the appearance of the spot indicating the presence of fats.
- How can you differentiate between fats and oils using the spot test?
- Discuss the significance of the spot test in food analysis.
Answer: (a) The principle is based on the fact that fats and oils leave translucent spots on paper due to their inability to evaporate. (b) The procedure involves spotting the sample onto filter paper and allowing it to dry. (c) A translucent spot is formed on the paper, indicating the presence of fats. (d) Fats leave a solid, opaque spot, while oils leave a translucent spot that may spread. (e) The spot test is significant as it provides a quick and simple method to identify the presence of fats, essential in food quality control and analysis.
Question 4: Emulsion Test
Explain the emulsion test for fats. Discuss the principle and observation of this test.
- What is the principle behind the emulsion test?
- Describe the procedure of the emulsion test.
- Explain the appearance of the emulsion indicating the presence of fats.
- How does the emulsion test differ from the spot test in terms of observation?
- Discuss the applications of the emulsion test.
Answer: (a) The principle is based on the fact that fats form stable emulsions with water. (b) The procedure involves shaking the sample with water vigorously and observing the formation of an emulsion. (c) A milky or cloudy appearance indicates the presence of fats in the emulsion. (d) Unlike the spot test, where fats leave spots on paper, the emulsion test shows a dispersed cloudy appearance in the water. (e) The emulsion test finds applications in various industries such as food processing, cosmetics, and pharmaceuticals for quality control and analysis.
Question 5: Biuret Test
Discuss the Biuret test for proteins. Explain the principle, reagents used, and interpretation of results.
- What are the reagents used in the Biuret test?
- Describe the principle behind the Biuret test.
- Explain the appearance of the solution indicating the presence of proteins.
- What is the chemical reaction involved in the Biuret test?
- Discuss the significance of the Biuret test in protein analysis.
Answer: (a) Reagents used are sodium hydroxide and copper(II) sulfate. (b) The principle is based on the formation of a violet color complex when proteins react with copper ions in alkaline solution. (c) The solution turns violet or purple in the presence of proteins. (d) Peptide bonds in proteins react with Cu²⁺ ions in alkaline solution to form a violet-colored complex. (e) The Biuret test is significant in protein analysis as it provides a simple and rapid qualitative test for the presence of proteins, commonly used in biochemical and clinical laboratories.
🔗 Other Useful Links
- News By Amurchem
- Free Web Development Course
- All-in-One Exam Prep Portal
- Articles by Amurchem
- Grade 12 Section
- Grade 11 Section
- Grade 10 Section
- Grade 09 Section
- Advanced Artificial Course
- Home and Online Tuition
- Labs By Amurchem
- Science Lectures By Amurchem
- Social Media Executive Course
© 2025 AmurChem. All rights reserved.






